To calculate result you have to disable your ad blocker first.
Weight of Acid Calculator - Equivalent Weight
Enter Molecular weight & Basicity of acid to find the equivalent weight of that acid
Weight of Acid Calculator
Weight of Acid Calculator is a tool that helps to find the weight of an acid using the molecular weight of the acid and the basicity of the acid.
What is meant by the Weight of Acids?
The weight of acid shows the mass of a specific amount of acid substance that is under consideration in laboratory tests or any research experiment.
In chemistry, the weight of a substance is a measure of the amount of matter it contains which is expressed in units such as grams (g) or kilograms (kg).
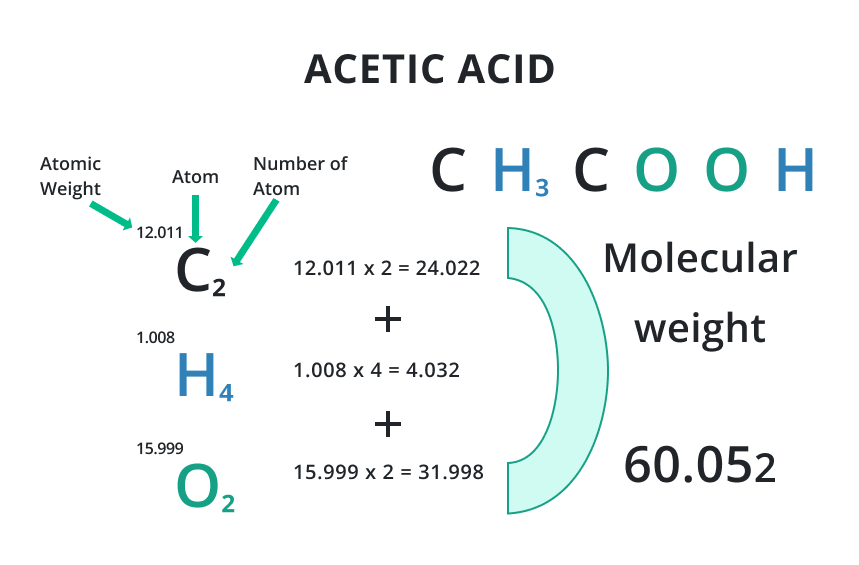
Acids are chemical compounds that typically have a sour taste and the ability to react with bases to produce salts in products with some impurities. The weight of an acid is determined by the mass of its constituent atoms and molecules.
Each acid has a specific molecular formula that represents the types and quantities of atoms present in the compound.
Formula:
Ew = Mw/B
Where,
- Ew = Equivalent weight of acid
- Mw = Molecular weight of acid
- B = Basicity of acid
How to evaluate the weight of Acid?
Example 1:
Find the weight of hydrochloric acid (HCl) if its molar mass or molecular weight is approximately 37 and its basicity is 24.
Solution:
Step 1: Write the data from the question.
Mw = 37, B = 24, Ew =?
Step 2: Write the formula of the weight of the acid.
Ew = Mw/B
Step 3: Put all values in the above formula and simplify.
Ew = 37/24
Ew = 1.543
Example 2:
Find the weight of Sulfuric Acid (H2SO4) if its molar mass or molecular weight is approximately 97 and its basicity is 54.
Solution:
Step 1: Write the data from the question.
Mw = 97, B = 54, Ew =?
Step 2: Write the formula of the weight of the acid.
Ew = Mw/B
Step 3: Put all values in the above formula and simplify.
Ew = 97/54
Ew = 1.796

