To calculate result you have to disable your ad blocker first.
Chemical Equation Balancer
To balance chemical equation, enter equation & hit calculate button using chemical equation balancer
Chemical Equation Balancer
The chemical equation balancer aids in maintaining the equilibrium on both sides of the equation. The calculator comes with the whole periodic table, making recognizing and inputting different elements very easy.
How to use this tool?
To use the chemical equation balancer, follow these steps.
- Enter the elements of the equation.
- Add the coefficients.
- Click Calculate.
You can click on any element of the periodic table to enter it or type in the element yourself.
What is an unbalanced chemical equation?
A chemical equation represents a chemical reaction with the help of symbols of the elements used in the reaction.
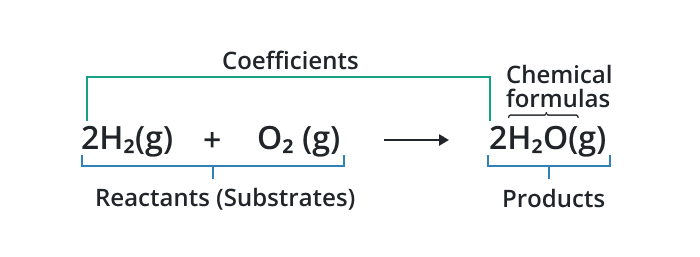
An unbalanced chemical equation is characterized by an unequal number of atoms on the reactant side compared to the product side. For example, consider the unbalanced equation for the combustion of methane:
CH₄ + O₂ → CO₂ + H₂O
In this equation, the number of atoms for each element is not balanced. On the left side, there is one carbon (C) atom and four hydrogen (H) atoms, while on the right side, there is one carbon (C) atom and only two hydrogen (H) atoms.
Similarly, on the left side, there are two oxygen (O) atoms, but on the right side, there are three oxygen (O) atoms.
Such equations go against the law of conservation of mass according to which matter can only change. It can neither be created nor destroyed.
How to balance chemical equations?
To satisfy the law of conservation of mass and obtain a balanced equation, the coefficients must be adjusted to ensure that the number of atoms for each element is the same on both sides.
Here is a general step-by-step guide on balancing chemical equations with examples.
Step 1: Write the unbalanced equation:
Begin by writing the chemical equation as it is, without any coefficients. For example, let's consider the combustion of methane:
CH₄ + O₂ → CO₂ + H₂O
Step 2: Count the number of atoms for each element:
Examine each element in the equation and count the number of atoms on both sides. Make a tally for each element:
Carbon (C): 1 on the left, 1 on the right
Hydrogen (H): 4 on the left, 2 on the right
Oxygen (O): 2 on the left, 3 on the right
Step 3: Start with the most complex or abundant element:
Begin balancing the equation by adjusting the coefficients for elements that appear in the fewest places or have the highest coefficient values. In this example, start with oxygen (O), as it appears in only one compound on each side.
Step 4: Use coefficients to balance atoms:
Add coefficients to the formulas of the compounds to balance the number of atoms for each element on both sides. To balance the oxygen atoms, add 2 as a coefficient in front of water(H₂O):
CH₄ + O₂ → CO₂ + 2H₂O
Step 5: Recount the number of atoms for each element:
After adding coefficients, recheck the number of atoms for each element on both sides of the equation:
Carbon (C): 1 on the left, 1 on the right
Hydrogen (H): 4 on the left, 4 on the right
Oxygen (O): 4 on the left, 4 on the right
Step 6: Continue balancing:
If the equation is still unbalanced, repeat steps 3 to 5, focusing on other elements that require balancing. In this example, carbon (C) and hydrogen (H) are already balanced, so move on to balance oxygen (O) in this case.
To balance the remaining oxygen atoms, place a coefficient of 2 in front of the oxygen (O₂) molecule:
CH₄ + 2O₂ → CO₂ + 2H₂O
Step 7: Check the final equation:
Once all the atoms are balanced, double-check that the equation is fully balanced, ensuring that the number of atoms for each element is equal on both sides:
Carbon (C): 1 on the left, 1 on the right
Hydrogen (H): 4 on the left, 4 on the right
Oxygen (O): 4 on the left, 4 on the right
How to balance chemical equations using the oxidation number method?
Balancing chemical equations using the oxidation number method involves assigning oxidation numbers to the elements in the reactants and products and then using the changes in oxidation numbers to balance the equation.
Here's a step-by-step guide on how to balance chemical equations using the oxidation number method:
Step 1: Write the unbalanced equation:
Consider the reaction between potassium permanganate (KMnO₄) and iron(II) sulfate (FeSO₄) to form manganese(II) sulfate (MnSO₄), iron(III) sulfate (Fe₂(SO₄)₃), and potassium sulfate (K₂SO₄):
KMnO₄ + FeSO₄ → MnSO₄ + Fe₂(SO₄)₃ + K₂SO₄
Step 2: Assign oxidation numbers:
Oxidation numbers are theoretical charges assigned to individual atoms within a compound, determined by a specific set of guidelines or rules.
- KMnO₄: K has a +1 oxidation number, Mn has a +7 oxidation number, and each oxygen (O) has a -2 oxidation number.
- FeSO₄: Fe has a +2 oxidation number, S has a +6 oxidation number, and each oxygen (O) has a -2 oxidation number.
- MnSO₄: Mn has a +2 oxidation number, S has a +6 oxidation number, and each oxygen (O) has a -2 oxidation number.
- Fe₂(SO₄)₃: Fe has a +3 oxidation number, S has a +6 oxidation number, and each oxygen (O) has a -2 oxidation number.
- K₂SO₄: K has a +1 oxidation number, S has a +6 oxidation number, and each oxygen (O) has a -2 oxidation number.
Step 3: Identify the elements undergoing oxidation and reduction:
The element that undergoes an increase in oxidation number is being oxidized, while the element that undergoes a decrease in oxidation number is being reduced.
In this example, the oxidation state of Mn in KMnO₄ changes from +7 to +2, indicating reduction. The oxidation state of Fe in FeSO₄ changes from +2 to +3, indicating oxidation.
Step 4: Balance the atoms undergoing oxidation and reduction:
In this case, we place a coefficient of 5 in front of FeSO₄ to balance the number of Fe atoms on both sides.
KMnO₄ + 5FeSO₄ → MnSO₄ + Fe₂(SO₄)₃ + K₂SO₄
Step 5: Balance the remaining atoms:
Place coefficients of 8, 1, and 10 in front of KMnO₄, MnSO₄, and K₂SO₄, respectively, to balance the remaining atoms.
8KMnO₄ + 5FeSO₄ → 8MnSO₄ + Fe₂(SO₄)₃ + 10K₂SO₄
Step 6: Verify the balance:
Double-check that the number of atoms of each element is the same on both sides of the equation. Also, ensure that the charges are balanced for any charged species present.
Step 7: Adjust coefficients as needed:
If the equation is not balanced yet, go back and adjust the coefficients as necessary until a balanced equation is achieved.
In this example, the final balanced equation for the reaction is:
8KMnO₄ + 5FeSO₄ → 8MnSO₄ + Fe₂(SO₄)₃ + 10K₂SO₄

